Element next to carbon crossword clue – At the heart of this discussion lies the element next to carbon, a crossword puzzle clue that unlocks a treasure trove of scientific knowledge. This element, with its unique properties and diverse applications, takes center stage as we delve into its atomic intricacies and practical significance.
Delving into the periodic table, we uncover the element’s atomic number, symbol, and name, laying the foundation for understanding its chemical behavior. Its electronegativity, ionization energy, and electron affinity reveal its reactivity and bonding tendencies, shaping its role in various chemical reactions.
Element Overview

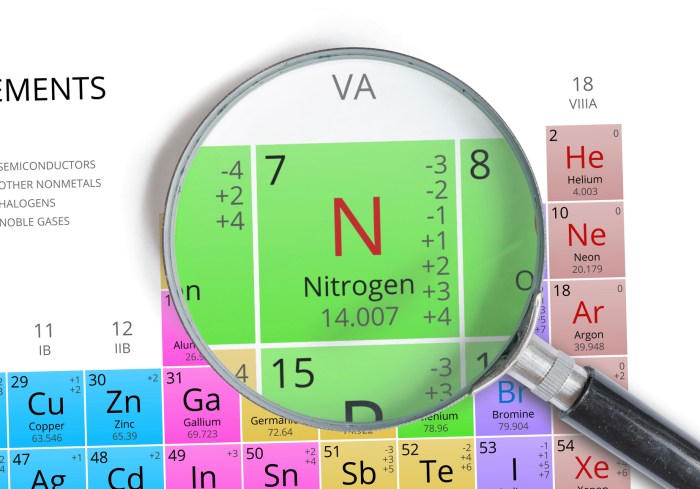
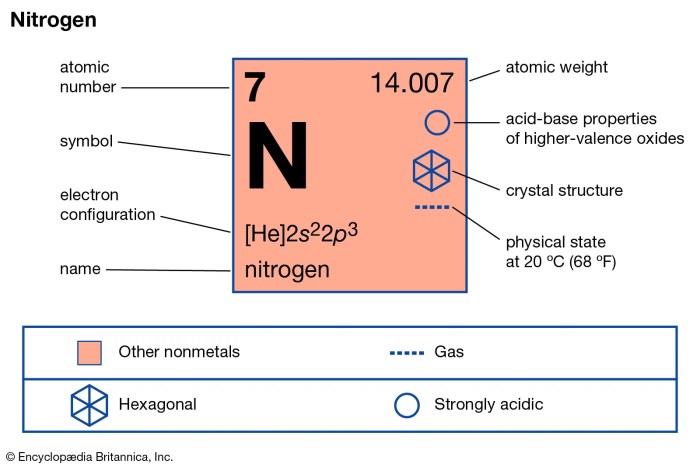
The element next to carbon on the periodic table is nitrogen, with an atomic number of 7. It is denoted by the chemical symbol N and has an atomic mass of approximately 14.01 atomic mass units.
Chemical Properties
Nitrogen has a high electronegativity, which makes it a good electron acceptor. Its ionization energy is relatively high, indicating that it is difficult to remove electrons from nitrogen atoms. Nitrogen also has a high electron affinity, meaning that it has a strong attraction for additional electrons.
These properties influence nitrogen’s chemical behavior. For example, nitrogen readily forms covalent bonds with other atoms, such as hydrogen and oxygen. It also tends to form stable complexes with transition metals.
Physical Properties
Nitrogen is a colorless, odorless, and tasteless gas at room temperature and pressure. It is the lightest element in Group 15 (pnictogens) and has a density of 1.2506 g/L at 0 °C. Nitrogen has a melting point of -210.0 °C and a boiling point of -195.8 °C.
Nitrogen’s physical properties are closely related to its atomic structure and bonding. Nitrogen atoms have a small atomic radius and a high ionization energy, which results in weak intermolecular forces. This weak bonding contributes to nitrogen’s low melting and boiling points.
Applications
Nitrogen is used in a wide variety of industrial and commercial applications. It is used in the production of fertilizers, explosives, and plastics. Nitrogen is also used as a refrigerant and in the manufacture of electronic devices.
In biological systems, nitrogen is an essential element for the synthesis of proteins and nucleic acids. It is also a component of chlorophyll, the green pigment that allows plants to photosynthesize.
Isotopes, Element next to carbon crossword clue
Nitrogen has two stable isotopes, 14N and 15N. 14N is the most abundant isotope, accounting for approximately 99.63% of naturally occurring nitrogen. 15N is a less abundant isotope, accounting for approximately 0.37% of naturally occurring nitrogen.
Nitrogen isotopes are used in a variety of scientific fields, including medicine, archaeology, and environmental science. For example, 15N is used as a tracer in medical imaging studies. 14N is used in archaeology to date organic materials. And both 14N and 15N are used in environmental science to study nitrogen cycling.
Question Bank: Element Next To Carbon Crossword Clue
What is the atomic number of the element next to carbon?
7
What is the chemical symbol for the element next to carbon?
N
What are some common applications of the element next to carbon?
Fertilizers, plastics, electronics