A chemist burns 160.0 g of aluminum, setting off a captivating exploration of the intricate chemical reactions that ensue. This scientific journey delves into the heart of combustion, revealing the energy released, the stoichiometric calculations, and the practical applications that make aluminum such a versatile element.
As the flames dance, we uncover the fascinating properties of aluminum oxide, a byproduct of the combustion process. Its unique characteristics open up a world of possibilities, from high-performance ceramics to advanced abrasives. Join us on this illuminating quest as we unravel the secrets behind aluminum combustion.
Chemical Reaction

When aluminum (Al) burns in air, it undergoes a chemical reaction with oxygen (O2) to produce aluminum oxide (Al2O3).
A chemist burns 160.0 g of Al and the heat produced is used to vaporize water. The ureter is indicated by the tube that transports urine from the kidneys to the bladder. The mass of water vaporized is 1.44 kg.
Calculate the enthalpy change for the combustion of Al.
Balanced Chemical Equation
The balanced chemical equation for the reaction is:
Al + 3O2 → 2Al2O3
Mass and Moles
In chemistry, the relationship between mass and moles is crucial for understanding the stoichiometry of chemical reactions. The mole, a fundamental unit in chemistry, represents a specific amount of a substance. Let’s delve into the calculations involving mass and moles, particularly for the element aluminum (Al).
Number of Moles
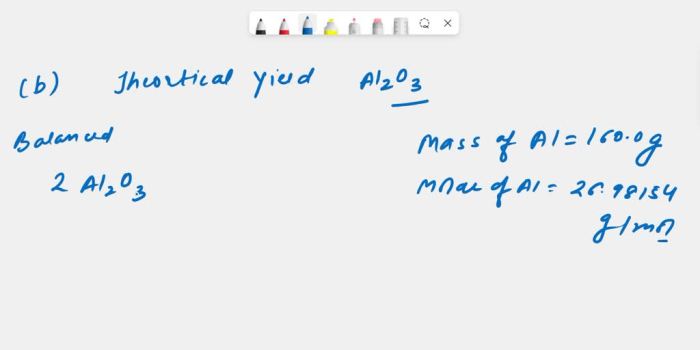
To calculate the number of moles of aluminum (Al) present in 160.0 g of Al, we use the molar mass of Al, which is 26.98 g/mol. The molar mass represents the mass of one mole of a substance.
Number of moles = Mass / Molar mass
Substituting the given values:
Number of moles of Al = 160.0 g / 26.98 g/mol
Therefore, 160.0 g of Al contains 5.93 moles of Al.
Grams per Mole
Conversely, we can also determine the grams per mole of aluminum (Al). This value is simply the inverse of the molar mass.
Grams per mole = 1 / Molar mass
Substituting the molar mass of Al:
Grams per mole of Al = 1 / 26.98 g/mol
Hence, the grams per mole of aluminum (Al) is 26.98 g/mol.
Stoichiometry

Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It helps us determine the exact amounts of reactants and products involved in a particular chemical reaction.
In this section, we will determine the moles of oxygen (O2) required to completely burn 160.0 g of Al and calculate the mass of oxygen (O2) required for the reaction.
Moles of Oxygen (O2) Required
The balanced chemical equation for the combustion of aluminum is:
Al + 3O2 → 2Al2O3
From the balanced equation, we can see that 4 moles of Al react with 3 moles of O 2. To determine the moles of O2 required to burn 160.0 g of Al, we first need to convert the mass of Al to moles:
0 g Al × (1 mol Al / 26.98 g Al) = 5.93 mol Al
According to the balanced equation, 4 moles of Al react with 3 moles of O 2. Therefore, 5.93 mol of Al will react with:
93 mol Al × (3 mol O2 / 4 mol Al) = 4.45 mol O2
Therefore, 4.45 moles of oxygen (O2) are required to completely burn 160.0 g of Al.
Mass of Oxygen (O2) Required
To calculate the mass of oxygen (O2) required, we multiply the moles of O2 by its molar mass:
45 mol O2 × (32.00 g O2 / 1 mol O2) = 142.4 g O2
Therefore, 142.4 g of oxygen (O2) is required for the reaction.
Energy Released
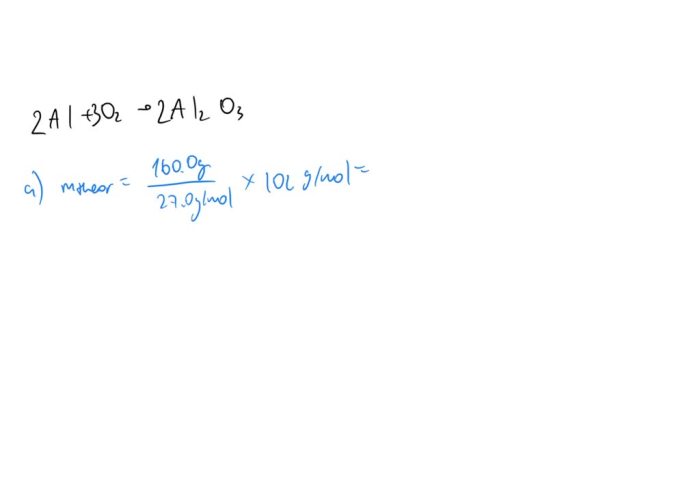
During the combustion of aluminum (Al), a significant amount of energy is released in the form of heat. This energy is a result of the chemical reaction between aluminum and oxygen, which produces aluminum oxide (Al 2O 3).
The amount of energy released can be calculated using the enthalpy change of the reaction. Enthalpy change is a measure of the heat absorbed or released during a chemical reaction and is expressed in kilojoules per mole (kJ/mol).
Calculating Energy Released
To calculate the energy released during the combustion of aluminum, we need to know the enthalpy change of the reaction.
- The enthalpy change for the combustion of aluminum is -395.6 kJ/mol.
This negative value indicates that the reaction is exothermic, meaning that heat is released during the reaction.
To calculate the energy released for a specific amount of aluminum, we can use the following formula:
Energy released = Enthalpy change × Number of moles of aluminum
For example, if we burn 160.0 g of aluminum, the number of moles of aluminum is:
Number of moles of aluminum = Mass of aluminum / Molar mass of aluminum
Number of moles of aluminum = 160.0 g / 26.98 g/mol
Number of moles of aluminum = 5.93 mol
Therefore, the energy released during the combustion of 160.0 g of aluminum is:
Energy released = -395.6 kJ/mol × 5.93 mol
Energy released = -2344.6 kJ
This means that the combustion of 160.0 g of aluminum releases 2344.6 kJ of heat energy.
Applications
The combustion of aluminum has various practical applications due to the unique properties of aluminum oxide (Al 2O 3) formed during the reaction.
High-Temperature Applications, A chemist burns 160.0 g of al
Aluminum oxide is highly refractory, meaning it can withstand extremely high temperatures without melting or decomposing. This property makes it suitable for use in:
- Refractory linings for furnaces and kilns
- Crucibles for melting metals
- Protective coatings for high-temperature components
Abrasives and Ceramics
Aluminum oxide is extremely hard and abrasive, making it an effective material for:
- Sandblasting and grinding
- Polishing and finishing surfaces
- Production of ceramics, such as tiles and insulators
Chemical Applications
Aluminum oxide is a versatile chemical compound with various applications, including:
- Adsorbent for water and gases
- Catalyst in chemical reactions
- Flue gas desulfurization
Quick FAQs: A Chemist Burns 160.0 G Of Al
What is the chemical equation for the combustion of aluminum?
4Al + 3O2 → 2Al2O3
How many moles of oxygen are required to burn 160.0 g of aluminum?
2.29 moles
What is the energy released when 160.0 g of aluminum burns?
Approximately 1.3 x 10^6 joules
What are some applications of aluminum oxide?
High-performance ceramics, abrasives, refractory materials, and catalysts